Types and Applications of Calcium Indicators for Calcium Imaging

Overview
Calcium ions play a crucial role within cells, and accurately measuring their dynamics is extremely important in cellular biology and neuroscience research.
However, many researchers may be unsure about which calcium indicator to use.
There are many different types of calcium indicators, including Fura-2, Fluo-4, and Rhod-2. Each indicator has different wavelength characteristics and dissociation constants, so it is necessary to use them appropriately depending on the specific research purpose and experimental conditions.
This article explains the types of reagents used in calcium imaging and how to choose them.
What are Calcium Indicators?
Calcium indicators are chemical substances used to measure the concentration of calcium ions (Ca2+) within cells. They are also known as fluorescent calcium dyes or calcium-sensitive dyes.
Calcium ions are essential for physiological processes such as muscle contraction and neural transmission. Calcium indicators emit fluorescence when they bind to calcium ions, allowing the visualization of calcium concentration changes.
Types of calcium indicators
There are several types of calcium indicators.
(1) Calcium-sensitive fluorescent dye using calcium chelating agent
These include Ca2+-selective chelators like EGTA and BAPTA. Examples of such dyes are Quin-2/AM, developed by R.Y. Tsien in 1980, and others like Fura-2, Fluo-3, Indo-1, and Rhod-2.
(2) Calcium-sensitive luminescent proteins such as Aequorin
These proteins emit weak light when they bind to Ca2+.
(3) Calcium-sensitive fluorescent proteins encoded by genes such as Cameleon and GCaMP (Genetically Encoded Calcium Indicator: GECI)
These use Ca2+-binding calmodulin to create fluorescent proteins.
This article focuses on calcium-sensitive fluorescent dyes and explains their principles and types.
Mechanism of Action of Calcium Indicators
Calcium indicators are primarily composed of chelators and fluorescent substance. When Ca2+ binds to the chelator part of the indicator, the fluorescent properties change, resulting in an increase in fluorescence intensity or a shift in the fluorescence spectrum.
BAPTA, for example, is a derivative of EGTA with a benzene ring replacing the methylene group. BAPTA has high Ca²⁺ selectivity similar to EGTA but is less affected by pH changes and has faster Ca²⁺ binding and release kinetics.
Mechanism of Fluorescence Generation
Fluorescent substances absorb light at specific wavelengths. This light energy lifts the electrons within the fluorescent material from the ground state to the excited state.
The absorbed energy moves the electron to a higher energy excited state. This state is unstable, and the electrons quickly lose energy and try to return to the ground state.
When the electrons return to their ground state, they emit part of the absorbed light energy as light. This emitted light typically has a longer wavelength and therefore lower energy than the absorbed light. This phenomenon is known as Stokes shift.
The emitted light is observed as fluorescence. The wavelength and intensity of fluorescence depend on the properties of the fluorophore and the surrounding environmental conditions.
The most efficient wavelengths for these transitions are the maximum excitation and emission wavelengths.

Types of calcium indicators
There are different types of calcium indicators. There are two main types: fluorescent indicators and absorption indicators, but the absorption type is currently not widely used.
Fluorescent types can be further divided into (1) those whose fluorescence intensity changes according to changes in ion concentration, and (2) those whose excitation or fluorescence spectrum changes according to changes in ion concentration.
(1) Fluorescence intensity changes according to changes in ion concentration
(1-1) 1-wavelength excitation / 1-wavelength fluorescence (e.g. Fluo-3, Fluo-4, Rhod-2, etc.)
Relative changes in calcium concentration can be inferred from changes in fluorescence intensity. This is often expressed as the change in fluorescence relative to the initial fluorescence (ΔF/F).
・Easy to measure because there is no need to change the wavelength and
measure as in two-wavelength measurement
・Because most of the excitation is visible light, there is no need for
an optical system that transmits ultraviolet light, and there is little
photodamage to cells.
Accurate measurement of changes in Ca2⁺ ion concentration becomes difficult when there are differences in concentration or uneven distribution of fluorescent dyes, changes in cell thickness, or severe bleaching of fluorescence due to strong excitation light.
(2) Excitation or fluorescence spectra change according to changes in ion concentration
(2-1) 2-wavelength excitation / 1-wavelength fluorescence (e.g. Fura-2)
(2-2) 1-wavelength excitation / 2-wavelength fluorescence (e.g. Indo-1)
Two wavelengths are used as excitation or fluorescence wavelengths.
・By taking the ratio of the fluorescence intensities of two
wavelengths, it is possible to correct fluctuations in fluorescence
intensity that are unrelated to changes in ion intensity (differences in
concentration, changes in cell thickness, etc.).
・By performing appropriate calibration, the absolute value of the ion
concentration can be determined.
・Requires special light sources and wavelength-splitting devices.
・Since ultraviolet light is used, an optical system that transmits
ultraviolet light is required. It also causes significant photodamage to
cells.
List of Major Calcium Indicators: High Affinity
| Calcium indicator | Excitation wavelength (nm) |
Emission wavelength (nm) |
Dissociation constant (Kd) |
Notes | Link |
|---|---|---|---|---|---|
| Calcium Green-1 | 506 | 531 | 190nM | single wavelength | https://www.thermofisher.com/order/catalog/product/C3011MP |
| Fluo-3 AM | 506 | 526 | 325nM | single wavelength | https://www.thermofisher.com/order/catalog/product/F1241 |
| Fluo-4 AM | 494 | 516 | 345nM | single wavelength | https://www.thermofisher.com/order/catalog/product/F14201 |
| Fluo-8 AM | 494 | 517 | 390nM | single wavelength | https://www.aatbio.com/products/fluo-8-am |
| Fluo-Gold AM | 525 | 550 | 400nM | single wavelength | https://ionbiosciences.com/store/fluo-gold-am/ |
| Fura-2 AM | 340, 380 | 510 | 224nM | ratiometry (dual excitation/ single emission) | https://www.thermofisher.com/order/catalog/product/F1221 |
| Fura Red AM | 420 / 480 or 457 / 488 | 660 | 200nM | ratiometry (dual excitation/ single emission) | https://www.thermofisher.com/order/catalog/product/F3020 |
| Indo-1 AM | 330-346 | 401 / 475 | 230nM | ratiometry (single excitation/ dual emission) | https://www.thermofisher.com/order/catalog/product/I1223 |
| Oregon green 488 BAPTA-1 | 488 | 520 | 170nM | single wavelength | https://www.thermofisher.com/order/catalog/product/O6807 |
| Quin-2 AM | 332-352 | 492 | 126nM | single wavelength | https://www.caymanchem.com/product/20422/quin-2-am |
| Rhod-2 AM | 533 | 576 | 570nM | single wavelength | https://www.thermofisher.com/order/catalog/product/R1245MP |
| X-Rhod-1 SM | 580 | 602 | 700nM | single wavelength | https://www.thermofisher.com/order/catalog/product/X14210 |
| Cal-520 AM | 494 | 514 | 320nM | single wavelength | https://www.aatbio.com/products/cal-520-am |
| Calbryte 520 AM | 492 | 514 | 1200nM | single wavelength | https://www.aatbio.com/products/calbryte-520-am |
| Calbryte 590 AM | 580 | 592 | 1400nM | single wavelength | https://www.aatbio.com/products/calbryte-590-am |
| Calbryte 630 AM | 608 | 624 | 1200nM | single wavelength | https://www.aatbio.com/products/calbryte-630-am |
| ICR-1 AM | 580 | 660 | 480nM | single wavelength | https://ionbiosciences.com/store/icr-1-am/ |
List of Major Calcium Indicators: Low Affinity
| Calcium indicator | Excitation wavelength (nm) |
Emission wavelength (nm) |
Dissociation constant (Kd) |
Notes | Link |
|---|---|---|---|---|---|
| Fluo-4FF AM | 494 | 516 | 9.7μM | single wavelength | https://www.thermofisher.com/order/catalog/product/F23981 |
| Fluo-5N AM | 494 | 516 | 90μM | single wavelength | https://www.thermofisher.com/order/catalog/product/F14204 |
| Rhod-5N | 551 | 576 | 320μM | single wavelength | https://www.thermofisher.com/order/catalog/product/R14207 |
Which Calcium-Sensitive Fluorescent Dye Should I Choose?
- Non-cytotoxicity.
- Sufficiently fast response rate compared to the changes in Ca2+ concentration to be measured
- Affinity for Ca2+ (Kd value) close to physiological levels
- Excitation light does not damage cells
- Excitation and fluorescence wavelengths of the reagent match the optical path characteristics of the microscope
- Minimal interference from other ions
Which calcium-sensitive fluorescent dye should I choose?
The fluorescent probe reagent must be introduced into the cells to be
measured, but because it is water-soluble, it cannot penetrate the cell
membrane as is.
Therefore, methods such as using modified
reagents that increase membrane permeability or directly injecting the
reagent into the cell are employed.
1. Microinjection
- Used in cells with low esterase activity
-- A microelectrode is inserted into the cell
-- Perfusion from a patch pipette
2. Introduction via Lipophilic Esters
- AM derivatives, which are commonly used, are created by esterifying the
probe reagent with an Acetoxymethyl (AM) group to make it lipophilic. The AM
derivative itself is non-fluorescent and has no binding ability with free
ions.
- Since it easily permeates the cell membrane, when cells are immersed in a
solution containing the AM derivative, it passes through the membrane and
enters the cell without binding to extracellular ions. Once inside, the AM
group is cleaved by esterases, allowing the probe to bind to ions and become
water-soluble, thus remaining in the cytoplasm. In tissue samples where
probe introduction is difficult, using surfactants like Cremophore EL can
sometimes be effective.
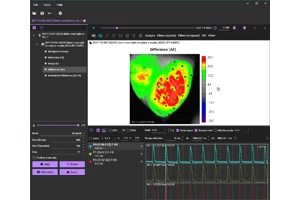
Sample data


Product suitable for this kind of application
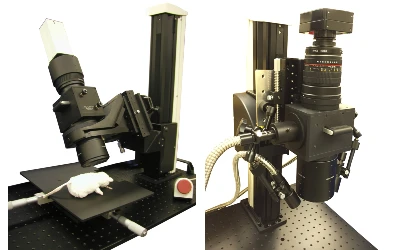
- High Speed Imaging System
- MiCAM03-N256
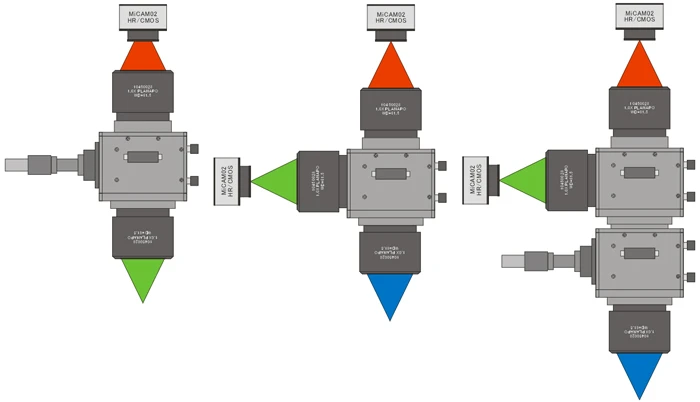
MiCAM03-N256 is a high-speed imaging system that captures and visualizes small changes in fluorescence intensity from biological samples stained with fluorescent probes, such as voltage sensitive dyes and calcium dyes.
- Spatial Resolution :
-
128x128 - 256x256 pixles
(32x32 pixels - 256x256 pixles with option) - Maximum Frame Rate :
- 1,000fps
(20,000 fps available with option) - Up to 2 camera heads can be used with completely synchronization.
- Fluorescence Mesoscope
- THT Series